
TOKYO, February 13, 2014 - Ajinomoto Co., Inc. (Ajinomoto Co.) and the Center for iPS Cell Research and Application at Kyoto University (Kyoto CiRA; Director: Professor Shinya Yamanaka, M.D.) have succeeded in joint development of StemFit® AK03, an iPS/ES cell culture medium with a higher level of safety, free of animal- and human-derived components, which is expected to be used in regenerative medicine. In addition, Ajinomoto Co. will supply the cell culture medium to Healios K.K., which aims to use these results to develop medical treatments with the world' s first retinal regenerative medicine using iPS cells.
○ Success in Developing a Cell Culture Medium with a Higher Level of Safety for Long-Term Passage Culture
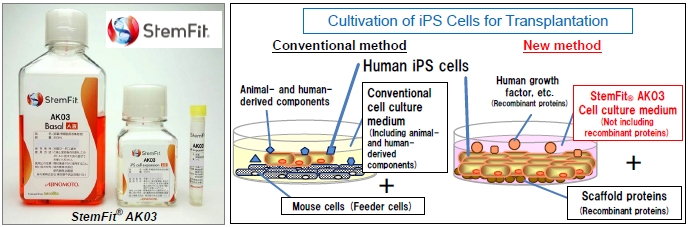
Since 2011, Ajinomoto Co. has been conducting joint research on cell culture media for cultivation of iPS cells with Dr. Masato Nakagawa, Lecturer, of Kyoto CiRA toward the realization of regenerative medicine using iPS cells. A cell culture medium is a nutrient solution for the cell proliferation. Until now, iPS cells has been cultured them with mouse cells called "feeder cells", using a culture medium that includes bovine serum.
By the novel technologies of Ajinomoto Co. and Kyoto CiRA' s expertise and research, the parties succeeded in developing StemFit® AK03 cell culture medium, which enables long-term passage culture, stable propagation of iPS cells and ES cells. As this cell culture medium is expected to be used in regenerative medicine, it uses recombinant proteins made with biotechnology to consist solely of refined substances completely free of animal- and human-derived components.
 ○ Consultation by PMDA
A consultation on the safety and the quality of StemFit® AK03 cell culture medium by the Pharmaceuticals and Medical Devices Agency, Japan (PMDA), the pharmaceutical regulatory and review agency of the Ministry of Health, Labour and Welfare, confirmed that the product does not include any raw materials to which the Standards for Biological Ingredients are applicable. Ajinomoto Co. believes this confirmation is important from the standpoint of safety.
○ Application in Clinical Research of the World' s First iPS-derived Regenerative Medicine
Ajinomoto Co. currently provides StemFit® AK03 cell culture medium to Kyoto CiRA. It also plans to supply the product to Healios K.K., which is conducting clinical research for a new treatment for age-related macular degeneration using the world' s first transplantation of iPS-derived retinal pigment epithelial cells in collaboration with Dr. Masayo Takahashi of RIKEN.
Ajinomoto Co. will promote development and industrialization of this cell culture medium, with plans to begin sales in 2016. Thereafter, with the expansion of regenerative medicine, Ajinomoto Co. aims for a total of JPY 40 billion in global sales of StemFit® AK03 and other cell culture media for regenerative medicine by 2025.
By marketing StemFit® cell culture medium for use in cultivation of iPS cells and ES cells, the Ajinomoto Group will contribute to the realization of regenerative medicine and the development of new pharmaceuticals, and thus to healthy human lives.
Note: The development of StemFit® cell culture medium was developed with the technologies of Professor Joseph Itskovitz-Eldor, M.D., D.Sc., of Technion - Israel Institute of Technology, under license from Accellta Ltd. of Israel.
Outline of Healios K.K.
Glossary
iPS cells (induced pluripotent stem cells)
Established by introducing a minute number of genes into ordinary human somatic (differentiated) cells such as skin cells. iPS cells can differentiate into various tissue and organ cells and proliferate indefinitely in culture.
ES cells (embryonic stem cells)
Established by removing cells from a 6-7 day old embryo and growing them in culture. ES cells can differentiate into any type of tissue cell in the body.
Recombinant proteins
Proteins manufactured with biotechnology using microorganism. Recombinant proteins are widely used for the therapy for cancer and rheumatism by biopharmaceuticals.
Standards for Biological Ingredients
Standards for raw materials or ingredients derived from humans or other organisms (with the exception of plants) when used in the production process for medical and pharmaceutical products.
About Ajinomoto Co.
Ajinomoto Co. is a global manufacturer of high-quality seasonings, processed foods, beverages, amino acids, pharmaceuticals and specialty chemicals. For many decades Ajinomoto Co. has contributed to food culture and human health through wide-ranging application of amino acid technologies. Today, the company is becoming increasingly involved with solutions for improved food resources, human health and global sustainability. Founded in 1909 and now operating in 26 countries and regions, Ajinomoto Co. had net sales of JPY 1,172.4 billion (USD 14.1 billion) in fiscal 2012. For more about Ajinomoto Co. (TYO: 2802), visit www.ajinomoto.com.
For further information, please contact here.
|
|||||||||||
| CLOSE |